Mutualism (biology)
Mutualism is the way two organisms of different species biologically interact in a relationship in which each individual derives a fitness benefit (i.e., increased or improved reproductive output). Similar interactions within a species are known as co-operation. Mutualism can be contrasted with interspecific competition, in which each species experiences reduced fitness, and exploitation, or parasitism, in which one species benefits at the expense of the other. Mutualism is a type of symbiosis. Symbiosis is a broad category, defined to include relationships that are mutualistic, parasitic, or commensal. Mutualism is only one type.
A well known example of mutualism is the relationship between ungulates (such as cows) and bacteria within their intestines. The ungulates benefit from the cellulase produced by the bacteria, which facilitates digestion; the bacteria benefit from having a stable supply of nutrients in the host environment.
Mutualism plays a key part in ecology. For example, mutualistic interactions are vital for terrestrial ecosystem function as more than 48% of land plants rely on mycorrhizal relationships with fungi to provide them with inorganic compounds and trace elements. In addition, mutualism is thought to have driven the evolution of much of the biological diversity we see, such as flower forms (important for pollination mutualisms) and co-evolution between groups of species.[1] However mutualism has historically received less attention than other interactions such as predation and parasitism.[2][3]
Measuring the exact fitness benefit to the individuals in a mutualistic relationship is not always straightforward, particularly when the individuals can receive benefits from a variety of species, for example most plant-pollinator mutualisms. It is therefore common to categorise mutualisms according to the closeness of the association, using terms such as obligate and facultative. Defining "closeness," however, is also problematic. It can refer to mutual dependency (the species cannot live without one another) or the biological intimacy of the relationship in relation to physical closeness (e.g., one species living within the tissues of the other species).[4]
Contents |
Types of relationships
Mutualistic transversals can be thought of as a form of "biological barter"[4] in which species trade resources (for example carbohydrates or inorganic compounds) or services such as gamete, offspring dispersal, or protection from predators.
Resource-resource relationships
Resource-resource interactions, in which one type of resource is traded for a different resource, are probably the most common form of mutualism; for example mycorrhizal associations between plant roots and fungi, with the plant providing carbohydrates to the fungus in return for primarily phosphate but also nitrogenous compounds. Other examples include rhizobia bacteria that fix nitrogen for leguminous plants (family Fabaceae) in return for energy-containing carbohydrates.[5]
Service-resource relationships
Service-resource relationships are also common.
Pollination in which nectar or pollen (food resources) are traded for pollen dispersal (a service) or ant protection of aphids, where the aphids trade sugar-rich honeydew (a by-product of their mode of feeding on plant sap) in return for defense against predators such as ladybird beetles.
Phagophiles feed (resource) on ectoparasites, thereby providing anti-pest service.
Zoochory is an example where animals disperse the seeds of plants. This is similar to pollination in that the plant produces food resources (for example, fleshy fruit, overabundance of seeds) for animals that disperse the seeds (service).
Service-service relationships
Strict service-service interactions are very rare, for reasons that are far from clear.[4] One example is the relationship between sea anemones and anemonefish in the family Pomacentridae: the anemones provide the fish with protection from predators (which cannot tolerate the stings of the anemone's tentacles) and the fish defend the anemones against butterflyfish (family Chaetodontidae), which eat anemones. However, in common with many mutualisms, there is more than one aspect to it: in the anemonefish-anemone mutualism, waste ammonia from the fish feed the symbiotic algae that are found in the anemone's tentacles.[6][7] Therefore what appears to be a service-service mutualism in fact has a service-resource component. A second example is that of the relationship between some ants in the genus Pseudomyrmex and trees in the genus Acacia, such as the Whistling Thorn and Bullhorn Acacia. The ants nest inside the plant's thorns. In exchange for shelter, the ants protect acacias from attack by herbivores (which they frequently eat, introducing a resource component to this service-service relationship) and competition from other plants by trimming back vegetation that would shade the acacia. In addition, another service-resource component is present, as the ants regularly feed on lipid-rich food-bodies called Beltian bodies that are on the Acacia plant.
In the Neotropics, the ant, Myrmelachista schumanni makes its nest in special cavities in Duroia hirsute. Plants in the vicinity that belong to other species are killed with formic acid. This selective gardening can be so aggressive that small areas of the rainforest are dominated by Duroia hirsute. These peculiar patches are known by local people as "devil's gardens".[8]
In some of these relationships, the cost of the ant’s protection can be quite expensive. Cordia sp. trees in the Amazonian rainforest have a kind of partnership with Allomerus sp. ants, which make their nests in modified leaves. To increase the amount of living space available, the ants will destroy the tree’s flower buds. The flowers die and leaves develop instead, providing the ants with more dwellings (houses). Another type of Allomerus sp. ant lives with the Hirtella sp. tree in the same forests, but in this relationship the tree has turned the tables on the greedy ants. When the tree is ready to produce flowers, the ant abodes on certain branches begin to wither and shrink, forcing the occupants to flee, leaving the tree’s flowers to develop free from ant attack.[8]
Humans and mutualism
Humans also engage in mutualisms with other species, including their gut flora (without which they would not be able to digest food efficiently) and domesticated animals such as horses, which provide transportation and other work in return for food and shelter.
In traditional agriculture, many plants will function mutualistically as companion plants, providing each other with shelter, soil fertility and/or natural pest control. For example, beans may grow up cornstalks as a trellis, while fixing nitrogen in the soil for the corn, a phenomenon that is used in Three Sisters farming.
Mathematical modeling
In 1989, David Hamilton Wright developed a mathematical explanation for mutualism using the Lotka–Volterra equation. Wright modified the Lotka-Volterra equations by adding a new term, βM/K, to represent a mutualistic relationship.[9]
The mutalistic relationship is quantified by:
where,
- N and M = the population density
- r = intrinsic growth rate of the population
- K = carrying capacity of its local environmental setting.
- β = coefficient converting encounters with one species to new units of the other
Mutualism is in essence the logistic growth equation + mutualistic interaction. The mutualistic interaction term represents the increase in population growth of species one as a result of the presence of greater numbers of species two, and vice versa. Wright also considered the concept of saturation, which means that with higher densities, there are decreasing benefits of further increases of the mutualist population. Without saturation, species' densities would increase indefinitely. Because that isn't possible due to environmental constraints and carrying capacity, a model that includes saturation would be more accurate. Wright's mathematical theory is based on the premise of a simple two-species mutualism model in which the benefits of mutualism become saturated due to limits posed by handling time. Wright defines handling time as the time needed to process a food item, from the initial interaction to the start of a search for new food items and assumes that processing of food and searching for food are mutually exclusive. Mutualists that display foraging behavior are exposed to the restrictions on handling time. Mutualism can be associated with symbiosis
Type II functional response
In 1959, C. S. Holling performed his classic disc experiment that assumed the following: that (1), the number of food items captured is proportional to the allotted searching time; and (2), that there is a variable of handling time that exists separately from the notion of search time. He then developed an equation for the Type II functional response, which showed that the feeding rate is equivalent to
where,
- a = the instantaneous discovery rate
- x = food item density
- TH = handling time
The equation that incorporates Type II functional response and mutualism is:
where,
- N and M = density of the two mutualists
- r = intrinsic rate of increase of N
- c = coefficient measuring negative intraspecific interaction
- X = 1/a TH
- β = b/ TH
- a = instantaneous discovery rate
- b = coefficient converting encounters with M to new units of N
Rearranged:
The model presented above is most effectively applied to free-living species that encounter a number of individuals of the mutualist part in the course of their existences. Of note, as Wright points out, is that models of biological mutualism tend to be similar qualitatively, in that the featured isoclines in general have a positive decreasing slope, and by and large similar isocline diagrams. Mutualistic interactions are best visualized as positively sloped isoclines, which can be explained by the fact that the saturation of benefits accorded to mutualism or restrictions posed by outside factors contribute to a decreasing slope.
See also
- Arbuscular mycorrhiza
- Co-adaptation
- Co-evolution
- Ecological facilitation
- Frugivory
- Greater Honeyguide - has an interesting mutualism with humans
- Interspecific communication
- List of symbiotic relationships
- Müllerian mimicry
- Mutualisms and Conservation
- Mutual Aid: A Factor of Evolution
- Symbiogenesis
Notes
- ^ Thompson, J. N. 2005 The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press.
- ^ Bronstein, JL. 1994. Our current understand of mutualism. Quarterly Review of Biology 69 (1): 31-51 March 1994
- ^ Begon, M., J.L. Harper, and C.R. Townsend. 1996. Ecology: individuals, populations, and communities, Third Edition. Blackwell Science Ltd., Cambridge, Massachusetts, USA.
- ^ a b c Ollerton, J. 2006. "Biological Barter": Patterns of Specialization Compared across Different Mutualisms. pp. 411-435 in: Waser, N.M. & Ollerton, J. (Eds) Plant-Pollinator Interactions: From Specialization to Generalization. University of Chicago Press.
- ^ Denison RF, Kiers ET 2004. Why are most rhizobia beneficial to their plant hosts, rather than parasitic. Microbes and Infection 6 (13): 1235-1239
- ^ Porat, D. & Chadwick-Furman, N. E. 2004 Effects of anemonefish on giant sea anemones: expansion behavior,growth, and survival. Hydrobiologia 530, 513–520. (doi:10.1007/s10750-004-2688-y)
- ^ Porat, D. & Chadwick-Furman, N. E. 2005 Effects of anemonefish on giant sea anemones: ammonium uptake,zooxanthella content and tissue regeneration. Mar. Freshw.Behav. Phys. 38, 43–51. (doi:10.1080/102362405000 57929)
- ^ a b Piper, Ross (2007), Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals, Greenwood Press.
- ^ Wright, David Hamilton. 1989. A Simple, Stable Model of Mutualism Incorporating Handling Time. The American Naturalist, Vol. 134, No. 4, pp. 664-667.
References
- Breton, Lorraine M., and John F. Addicott. 1992. Density-Dependent Mutualism in an Aphid-Ant Interaction. Ecology, Vol. 73, No. 6, pp. 2175–2180.
- Bronstein, JL. 1994. Our current understanding of mutualism. Quarterly Review of Biology 69 (1): 31-51 March 1994
- Bronstein JL. 2001. The exploitation of mutualisms. Ecology Letters 4 (3): 277-287
- Bronstein JL. 2001. The costs of mutualism. American Zoologist 41 (4): 825-839 S
- Bronstein JL, Alarcon R, Geber M. 2006. The evolution of plant-insect mutualisms. New Phytologist 172 (3): 412-428
- Denison RF, Kiers ET. 2004. Why are most rhizobia beneficial to their plant hosts, rather than parasitic? Microbes and Infection 6 (13): 1235-1239 ISSN 1286-4579
- DeVries, PJ; and Baker, I. 1989. Butterfly exploitation of an ant-plant mutualism: Adding insult of herbivory. Journal of the New York Entomological Society [J. N.Y. ENTOMOL. SOC.]. Vol. 97, no. 3, pp. 332–340. ISSN 0028-7199
- Hoeksema, J.D. & E.M. Bruna. 2000. Pursuing the big questions about interspecific mutualism: a review of theoretical approaches. Oecologia 125:321-330 ISSN 0029-8549
- Jahn, G.C. and J.W. Beardsley. 2000. Interactions of ants (Hymenoptera: Formicidae) and mealybugs (Homoptera: Pseudococcidae) on pineapple. Proceedings of the Hawaiian Entomological Society 34: 181-185. ISSN 0073-134X
- Jahn, Gary C., J. W. Beardsley and H. González-Hernández 2003. A review of the association of ants with mealybug wilt disease of pineapple. Proceedings of the Hawaiian Entomological Society. 36:9-28.ISSN 0073-134X
- Noe, R. & P. Hammerstein. 1994. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behavioral Ecology and Sociobiology 35:1-11 ISSN 0340-5443
- Ollerton, J. 2006. "Biological Barter": Patterns of Specialization Compared across Different Mutualisms. pp. 411–435 in: Waser, N.M. & Ollerton, J. (Eds) Plant-Pollinator Interactions: From Specialization to Generalization. University of Chicago Press. ISBN 9780226874005
- Paszkowski, U. 2006. Mutualism and parasitism: the yin and yang of plant symbioses. Current Opinion in Plant Biology 9 (4): 364-370. (doi:10.1016/j.pbi.2006.05.008. PMID 16713732
- Porat, D. & Chadwick-Furman, N. E. 2004. Effects of anemonefish on giant sea anemones:expansion behavior, growth, and survival. Hydrobiologia 530, 513–520. (doi:10.1007/s10750-004-2688-y)
- Porat, D. & Chadwick-Furman, N. E. 2005. Effects of anemonefish on giant sea anemones: ammonium uptake,zooxanthella content and tissue regeneration. Mar. Freshw. Behav. Phys. 38, 43–51. (doi:10.1080/102362405000 57929)
- Thompson, J. N. 2005. The Geographic Mosaic of Coevolution. University of Chicago Press. ISBN 978-0-226-79762-5
- Wright, David Hamilton. 1989. A Simple, Stable Model of Mutualism Incorporating Handling Time. The American Naturalist, Vol. 134, No. 4, pp. 664–667.
Further reading
- Boucher, D. G., James, S. & Kresler, K. (1984) The ecology of mutualism. Annual Review of Ecology and Systematics, 13: 315-347.
- Boucher, D. H. (editor) (1985) The Biology of Mutualism : Ecology and Evolution London : Croom Helm 388 p. ISBN 0-7099-3238-3
|
|||||
|
||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
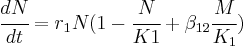
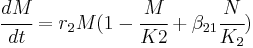
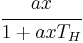
![\cfrac{dN}{dt}=N[r(1-cN)%2B\beta M(X%2BM)]](/2012-wikipedia_en_all_nopic_01_2012/I/f83f4e5a3d3c197a2462ae987cc0e07e.png)
![\cfrac{dN}{dt}=N\bigg[r(1-cN)%2B\cfrac{baM}{1%2BaT_H M}\bigg]](/2012-wikipedia_en_all_nopic_01_2012/I/6fe4ba1dcdb4b28c0bbe4afa90d492f3.png)